EU legislation
A strong foundation in EU law
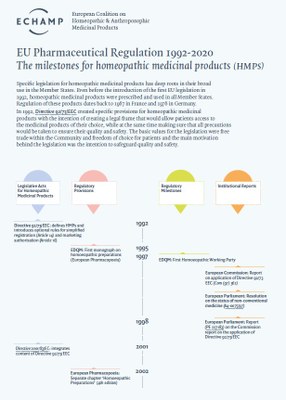
- Specific EU legislation for homeopathic medicinal products has deep roots in their broad use in the Member States.
- Most Member States officially confirm a market in their country and patients and prescribers use them in all Member States.
- Legislation is based on the EU principles of free trade and freedom of choice.
- The European Pharmacopoeia and other national pharmacopoeias determine the quality standards for the starting materials and manufacture of these products.
Specific EU legislation for homeopathic medicinal products has deep roots in their broad use in the Member States.
The legal basis
The authorisation for a homeopathic medicinal product for human use to be present on the market in the EU is regulated by specific provisions in Directive 2001/83/EC on medicinal products for human use, completed with specific provisions on the proof of quality, safety and efficacy in Directive 2003/63/EC.
Additional legislation of relevance to the legal and regulatory environment for these products includes:
- Directive 2004/24/EC introduces rules on ‘traditional herbal medicinal products’
- Directive 2004/27/EC provides general amendments
- Directive 2009/53/EC introduces rules on variations to the terms of marketing authorisations for medicinal products
- Directive 2011/62/EU prevents entry into the legal supply chain of falsified medicinal products
- Directive 2010/84/EU and Directive 2012/26/EU provide legislation on pharmacovigilance